Radical Deoxyfunctionalisation Strategies
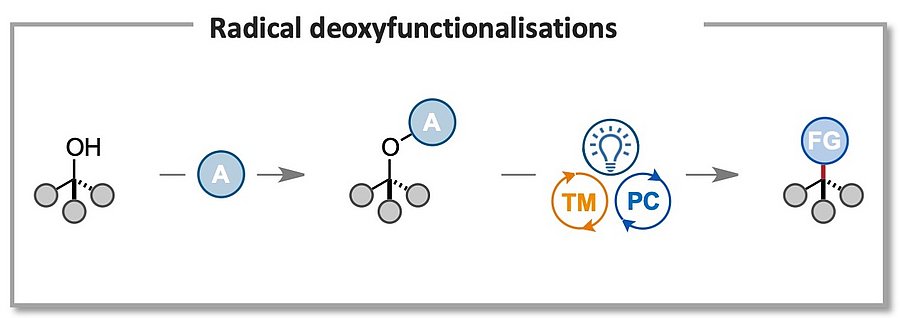
Due to their abundance and readily available synthesis, tertiary alcohols provide ideal handles for the selective derivatisation of organic molecules. In addition, hydroxy groups are ubiquitous structural motifs in biomolecules, such as steroids, saccharides, nucleosides, and therefore methodologies exploiting this chemical handle to achieve chemo- and regioselective direct derivatisations are of significant value. Our goal is to develop versatile radical-based methodologies that allow for the easy conversion of C–O bonds to C–X bonds (X = F, CN, N3, SCF3, etc), as well as selective late modifications of saccharides and nucleotides. This will help to expand the available chemical space by granting rapid access to libraries of unexplored chemical scaffolds!
Selected publications
3. Deoxygenative, Intramolecular Minisci-Type Reaction to Access Fused Heterocyclic Scaffolds; F. J. Aguilar Troyano,† K. Anwar,† F. Mohr, G. Robert, A. Gómez-Suárez* Eur. J. Org. Chem. 2022, e202201176 (†These authors contributed equally)
2. Radical deoxyfunctionalisation strategies; K. Anwar, K. Merkens, F. J. Aguilar Troyano, A. Gómez-Suárez* ChemRxiv DOI:10.26434/chemrxiv-2022-w73zr
1. Light‐mediated Formal Radical Deoxyfluorination of Tertiary Alcohols via Selective Single Electron Oxidation with TEDA2+·; F.J. Aguilar Troyano, F. Ballaschk,† M. Jaschinski,† Y. Ozkaya,† A. Gómez-Suárez* Chem. Eur. J. 2019, 25, 14054 (†These authors contributed equally)
